About
Executive Summary
Memorial Sloan Kettering (MSK) operates one of the largest cancer clinical trials programs in the United States, offering nearly 2,000 IRB-approved prospective protocols to patients, both for therapeutic and non-therapeutic purposes Our portfolio is further strengthened by clinical research opportunities led by pharmaceutical companies, federal agencies, consortia, and more. The ability to collaborate with these organizations and present their clinical studies to the MSK community depends heavily on efficient information and document exchange. Currently, this process primarily relies on point-to-point email communication, resulting in delays and making it difficult to track the number of studies requiring amendments at any given time.
Background
The current process for sharing protocol documents and processing amendments in clinical research is inefficient for both internal and external stakeholders. To address this, a need has been identified for centralization and standardization to streamline processing times and establish a consistent method of document exchange and communication. In response, MSK has developed PowerDOCX, a solution that will serve as a centralized hub for document exchange. Additionally, PowerDOCX functions as an automated, intelligent system that initiates the review and operationalization of amendment documentation before it even enters the work queue of an MSK staff member.
Features
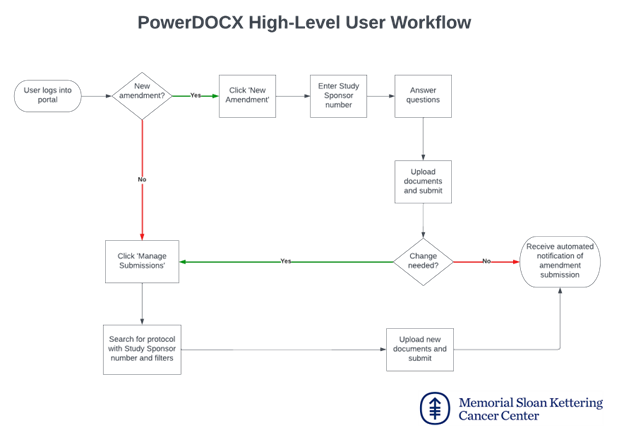
PowerDOCX will include several features designed to streamline the amendment process and improve transparency throughout the amendment lifecycle. The following features will be available to sponsors and Clinical Research Organizations (CROs) when submitting amendments via PowerDOCX:
1. New Amendment Creation – Users can initiate new amendment submissions by answering a series of questions and uploading the necessary documentation to process the request.
2. Uploading Additional Documentation – After creating an amendment, users can view the submission and upload any additional documents related to it. They can also "expire" a document, indicating that MSK study staff should no longer consider it the most current version.
3. Tracking Statuses – Sponsors and CROs can track the status of their amendments, such as whether MSK study staff have begun working on it, whether the Medicare Coverage Analysis review is complete, whether it has been submitted to the IRB, or if there are any obstacles delaying progress. A detailed historical record of the amendment’s lifecycle—from submission to completion—will also be available.
4. Automated Notifications – Users will receive automated notifications at each step of the process. This includes confirmation emails upon new amendment creation, notifications for any status updates, and alerts when there are blockers preventing progress.
5. Sharing Submissions – Sponsors can share their amendment submissions with other users within their organization. As long as the recipient has a registered PowerDOCX account, they can view the submission, upload new documentation, and track its status—even if they were not the original submitter.
Success Metrics
Currently, amendment submissions are not tracked or accounted for, so the immediate key benefit of PowerDOCX will be its ability to easily identify and quantify both submitted and in-progress amendments. This will enable MSK to set internal benchmark targets and prioritize submissions that are outside of typical processing times. In addition, PowerDOCX will enhance the turnaround time for processing study amendments. One of the most common causes of delay in the current workflow is the submission of incomplete protocol documentation. For example, amendments are sometimes submitted without all the necessary documents, which leads to delays in communication and the inability to implement the associated changes. By measuring the time taken to complete amendments before and after the implementation of PowerDOCX, MSK will be able to assess whether the system is effectively reducing amendment processing times.
Conclusion and Next Steps
With the anticipated benefits of a centralized document management and tracking system, we expect to reduce manual effort while improving amendment processing times and establishing a defined workflow for handling amendments across the organization. Our next steps involve collaborating with sponsors to onboard protocols and transition all new amendment submissions to the PowerDOCX platform. We will also continue to work closely with Clinical Research staff at MSK to ensure that PowerDOCX aligns with best practices and integrates seamlessly with current workflows, helping to streamline the overall backlog of amendments at MSK.


